Unidirectional Air Flow
Ensuring contamination-free environments with precise airflow control for cleanroom and high-grade sterile applications.
What is Unidirectional Air Flow
Unidirectional Air Flow
When a Unidirectional Air Flow (UDAF) system is connected to an Air Handling Unit (AHU) and used as a single pass-through system, it typically refers to a setup commonly found in controlled environments such as cleanrooms, laboratories, or certain manufacturing injectable aseptic filling processes
Unidirectional Air Flow (UDAF) System:
It creates a controlled airflow pattern where air moves in a single direction within a defined space. It maintains a clean environment over a filling line for pharmaceutical manufacturing by removing particles and contaminants.
Air Handling Unit (AHU):
An AHU conditions, filters, and circulates air within a building or controlled environment. In this context, the AHU provides the filtered and conditioned air that the UDAF system utilizes. It ensures the quality of the air supplied to the UDAF and subsequently to the filling line.

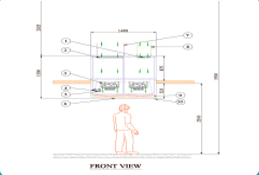
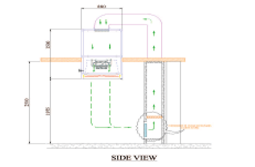
Single Pass-Through System:
The air passes through the UDAF and the controlled environment only once before being exhausted or possibly recirculated. This setup is used when maintaining a specific level of cleanliness is necessary and recirculating the air might carry contaminants back into the controlled environment.
Building Management System (BMS):
It is a centralized system that monitors and controls various building functions, including HVAC (Heating, Ventilation, and Air Conditioning) systems. In your case, the BMS controls the AHU and possibly the UDAF system as well. This helps to keep optimal conditions, efficiency, and coordination between different systems in the facility
In summary, your described scenario involves using a Unidirectional Air Flow (UDAF) system connected to an Air Handling Unit (AHU) in a single pass-through system over a filling line. A Building Management System (BMS) controls and coordinates this setup to maintain a clean and controlled environment crucial for sensitive processes. This method ensures the safety and quality of products in pharmaceutical or electronics manufacturing

What is Unidirectional Air Flow? A Deep Dive into Pharma Cleanroom Airflow and Laminar Airflow Systems
In industries like pharmaceuticals, biotechnology, and healthcare, maintaining a contaminant-free environment is critical. The integrity of products, patient safety, and regulatory compliance all hinge on precise control of air quality. This is where Unidirectional Air Flow (UDAF) plays a crucial role. In this blog, we will explore what is unidirectional air flow, why it is essential in pharma cleanroom airflow, the functioning of a laminar airflow system, and the best cleanroom airflow solutions available today.
What is Unidirectional Air Flow?
Unidirectional Air Flow refers to a controlled airflow pattern where air moves uniformly in a single direction, either vertically or horizontally, with minimal turbulence. This steady, laminar movement ensures airborne particles are swept away efficiently, reducing the risk of contamination within a cleanroom or controlled environment.
Unlike turbulent or mixed airflow systems where air moves randomly, unidirectional airflow creates a “sweeping” motion that continuously pushes contaminants out of critical zones. The concept of unidirectional air flow is fundamental in maintaining air purity, especially in sensitive environments such as pharmaceutical cleanrooms, semiconductor fabs, and hospitals.
The Importance of Pharma Cleanroom Airflow
In pharmaceutical manufacturing, maintaining a contamination-free environment is not just best practice — it’s mandatory. The pharma cleanroom airflow system is designed to ensure that airborne contaminants, dust particles, and microbes do not come into contact with drug products during production.
Cleanrooms must comply with stringent standards such as ISO 14644-1 and GMP guidelines. The right airflow system directly impacts these compliance levels. Pharma cleanroom airflow systems often rely on unidirectional airflow patterns to maintain ISO Class 5 or better cleanroom classifications.
Key reasons why pharma cleanroom airflow is critical include:
Product Safety: Ensures drugs are manufactured in sterile environments to prevent contamination.
Regulatory Compliance: Meets FDA and international regulatory bodies’ standards.
Operational Efficiency: Minimizes batch failures and costly recalls due to contamination.
Personnel Safety: Protects workers from exposure to hazardous substances.
How Does a Laminar Airflow System Work?
A laminar airflow system is a type of unidirectional airflow that moves filtered air at a uniform velocity in a single direction. The term “laminar” signifies smooth, layered air movement with little to no cross currents or eddies.
Components of a Laminar Airflow System:
HEPA or ULPA Filters: These filters remove particles as small as 0.3 microns with an efficiency of 99.97% or higher.
Air Handling Unit: Draws in ambient air, filters it, and supplies it to the cleanroom.
Airflow Diffusers: Direct the filtered air uniformly in the desired direction (vertical or horizontal).
Return Air Grilles: Collect contaminated air and recirculate or exhaust it.
Types of Laminar Airflow:
Vertical Laminar Airflow: Air is supplied from ceiling filters and moves downward, exiting through floor-level vents. Common in pharmaceutical cleanrooms and surgical suites.
Horizontal Laminar Airflow: Air enters from sidewall filters and exits through opposite side walls. Often used in clean benches and laboratories with overhead equipment restrictions.
The steady, unidirectional air stream helps sweep particles away from critical areas, reducing contamination risks.
Cleanroom Airflow Solutions: Tailoring Air Quality Control
Choosing the right cleanroom airflow solutions depends on the specific requirements of the controlled environment. Different industries and cleanroom classifications require tailored airflow systems to maintain air purity, safety, and compliance.
Factors to Consider in Cleanroom Airflow Solutions:
Cleanroom Classification: Higher-class cleanrooms (ISO Class 1–5) require stricter airflow control like unidirectional airflow.
Air Change Rate: Number of times the total volume of air is replaced per hour, critical for maintaining clean air standards.
Room Layout and Size: Influences whether vertical or horizontal airflow is more effective.
Operational Workflow: Airflow direction should complement personnel and material movement to minimize contamination.
Energy Efficiency: Modern solutions incorporate energy-saving technologies without compromising air quality.
Popular Cleanroom Airflow Solutions:
Unidirectional Airflow (UDAF): For critical zones requiring high contamination control.
Non-Unidirectional or Turbulent Airflow: Used in support zones where less strict controls are sufficient.
Hybrid Systems: Combine vertical and horizontal airflow for optimized performance.
Benefits of Unidirectional Air Flow in Pharma and Cleanrooms
Implementing unidirectional air flow and laminar airflow systems in pharma cleanroom airflow setups offers numerous benefits:
Superior Contamination Control: Reduces airborne particles and microbial contamination effectively.
Regulatory Compliance: Helps maintain adherence to international standards like GMP and ISO 14644.
Operational Reliability: Improves batch success rates by providing a consistent sterile environment.
Worker Safety: Minimizes exposure to hazardous materials through controlled airflow paths.
Energy Efficiency: Advanced designs optimize air handling, reducing energy consumption.
Best Practices for Maintaining Effective Cleanroom Airflow
To ensure that cleanroom airflow solutions perform optimally, consider the following best practices:
Regular Filter Maintenance: Replace HEPA/ULPA filters as per manufacturer guidelines.
Airflow Validation: Conduct smoke tests and particle count monitoring to verify laminar flow patterns.
Avoid Obstructions: Ensure equipment and personnel do not block airflow paths.
Gowning Procedures: Train staff on appropriate cleanroom protocols to maintain airflow integrity.
Continuous Monitoring: Use sensors to track airflow velocity and particulate levels in real time.
Conclusion
Understanding what is unidirectional air flow and its importance in pharma cleanroom airflow is vital for pharmaceutical manufacturers and other industries requiring stringent contamination control. The laminar airflow system remains one of the most effective methods to maintain sterile, particle-free environments, ensuring product quality and safety.
By implementing the right cleanroom airflow solutions tailored to your operational needs, you can achieve regulatory compliance, enhance product integrity, and protect both personnel and processes.
For businesses looking to design, upgrade, or validate cleanroom airflow systems, expert consultation and engineering services can ensure a seamless and effective implementation.
Table of Contents
Toggle

